BAT Levels Timeline: History of Alzheimer’s Disease & the Rise of BATWatch
Understanding brain health and the origins of Alzheimer’s disease requires more than just today’s research. At BATWATCH™, we believe that innovation stands on the shoulders of every breakthrough that came before it. That’s why our science and clinical teams have gone all the way back to the earliest days of Alzheimer’s research, gathering every milestone, study, and hypothesis from the historical archives to the latest peer-reviewed literature.
We’ve systematically collected, digitized, and analyzed over a century of scientific progress: from Dr. Alois Alzheimer’s first case report in 1907, to the discoveries of beta-amyloid and tau in the 1980s, through the biomarker revolution and today’s advances in plasma-based early detection.
By training our advanced AI models on this vast historical and contemporary data set, we’re able to spot patterns, validate protocols, and push the boundaries of brain health science, turning a century of discovery into actionable, preventive care.
Every data point, every study, and every landmark you’ll see below feeds directly into the BATWATCH™ Evidence Matrix – the living intelligence engine behind our patient protocols.
Want the full backstory behind these breakthroughs? Read the complete history of Alzheimer’s disease and the rise of BATWatch.
Our timeline below isn’t just a chronology, it’s the foundation of the BATWatch™ Protocol.
Explore how over a century of discovery has shaped the fight to end Alzheimer’s disease, through science, biomarkers, and the dawn of next-generation brain health prevention.

‘Alzheimer’s disease’

Figure 3. (Left to right) A. Alzheimer, E. Kraepelin, R. Gaupp, and F. Nissl. About 1906. © Archive for History of Psychiatry, Department of Psychiatry University of Munich. With permission.
Kraepelin E. Psychiatrie. 8th ed. Vol I: Allgemeine Psychiatrie; Vol II: Klinische Psychiatrie. Leipzig, Germany: Barth; 1909/1910 [Google Scholar]

Figure 1. Dr. George G. Glenner 1984. © George G. Glenner Alzheimer's Family Centers, Inc
Glenner GG & Wong CW (1984): Purified and characterized beta-amyloid as the core component of amyloid plaques in AD brains. PubMed
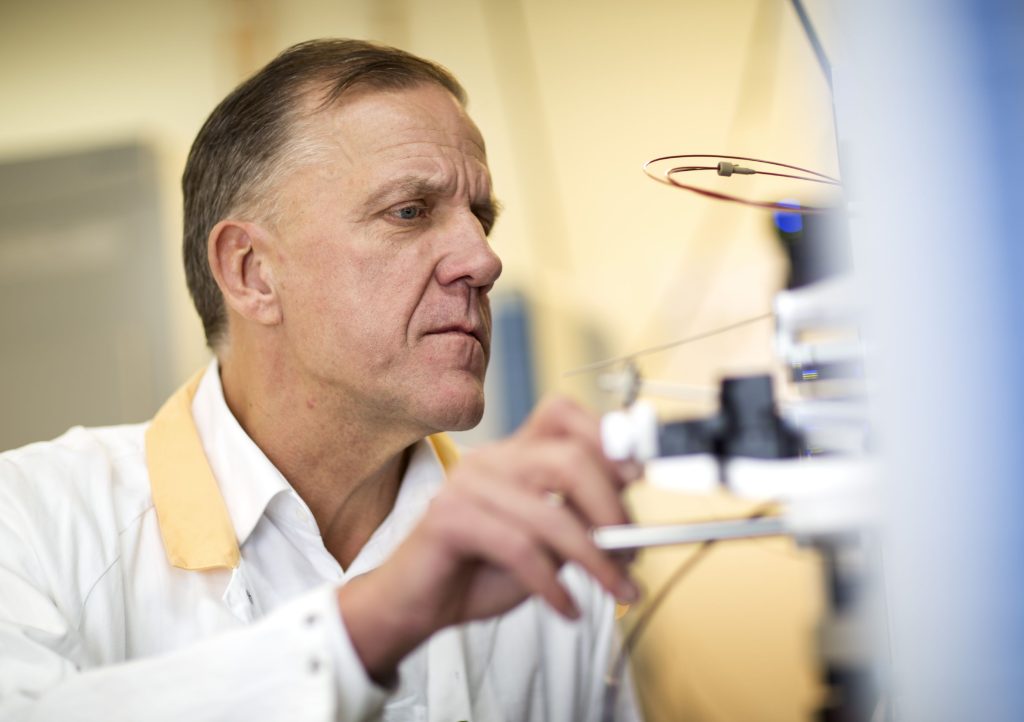
Figure 1. Dr. Kaj Blennow 2023. © Johan Wingborg, University of Gothenburg
Blennow, K., Wallin, A., et al. (1995): Staging of alzheimer's disease-related neurofibrillary changes. PubMed
gold-standard for Alzheimer's Risk

Figure 1. Niels Andreasen MD 2004. © ResearchGate GmbH.
in aging brain models (animal studies).

Figure 1. Patricia R. Spilman, M.A. 2014. © Regents of University of California
Spilman, P., et al. (2010): Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer's disease. PubMed
guidelines for early brain health monitoring

Figure 1. Dr. Reisa Sperling 2024. © Board of Regents of the University of Wisconsin
Sperling, R. A., Jack, C. R., Aisen, P. S., et al. (2011): Toward defining the preclinical stages of Alzheimer's disease: PubMed
accurate and accessible for routine brain
health monitoring

Figure 1. Sebastian Palmqvist, MD, PhD 2024. © Tove Smeds | American Psychiatric Association
Palmqvist, S., Janelidze, S., Stomrud, E., et al. (2019):Performance of Fully Automated Plasma Assays as Screening Tests for Alzheimer Disease–Related β-Amyloid Status: JAMA

removes analysis paralysis.

600 completed BAT Pill protocol.
surpasses 200 providers, throughout USA
and Canada.
